
Part 1: Surface Energy vs. Surface Tension
The effects of a solid object when in contact with a fluid generate different reactions that relate to the morphology and molecular structure at both a microscopic and macroscopic level. These factors include the surface roughness of the substrate and the intermolecular forces within the fluid – the hydrogen bonds and Van der Waals forces – that give us a good first approximation of potential bonding capability between an adhesive and solid. Since many of these vital characteristics are found at the molecular level, it is somewhat difficult to anticipate what an ideal adhesive would look like. The ability of an adhesive to spread across a surface (also known as wetting) is probably the best and easiest way to identify a suitable adhesive for a specific substrate. Ideally, a substrate with high surface energy paired with an adhesive with low surface tension will have good wetting characteristics, as shown in figure 1 below.
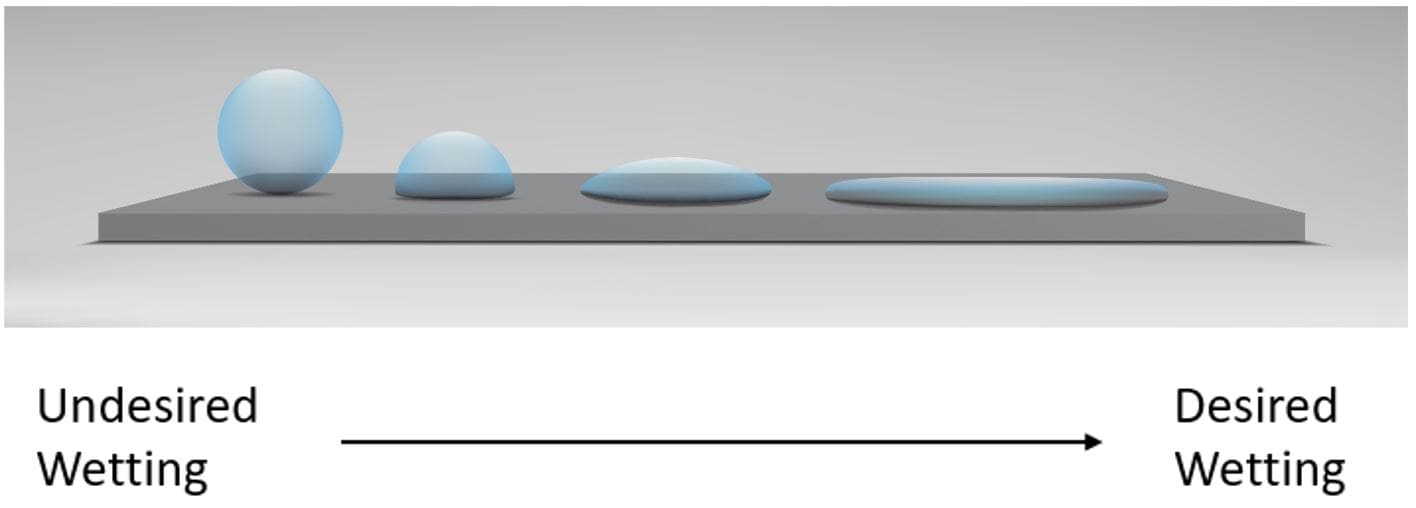
Contact angle goniometry is a technique that measures the contact angle between fluid (the adhesive) and the solid (the substrate). A smaller contact angle correlates to a higher degree of wetting. As a general rule, when 10˚< Ɵ < 90˚, there is favourable wetting, while angles where Ɵ > 90˚ lead to poor wetting.
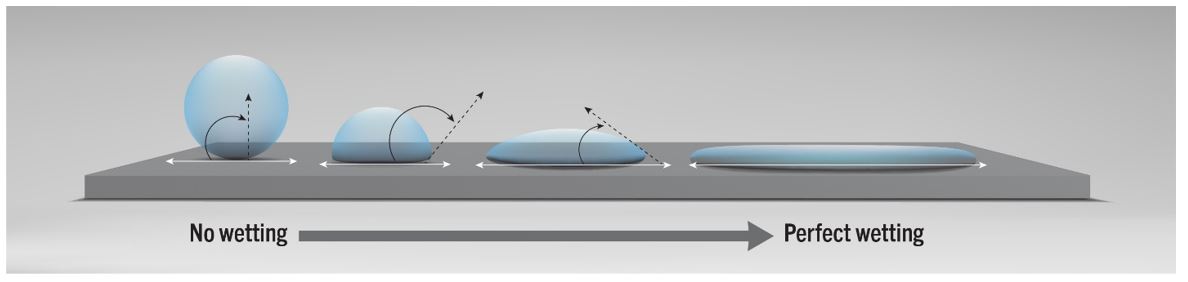
Good wetting is achieved with an adhesive having the right molecular structure and morphology and from proper surface preparation, where the substrate is properly cleaned and further treated to raise the overall surface energy. Part 2 of this white paper will address surface pre-treatment techniques used to improve the wetting of adhesives and subsequent adhesion.
Part 2: Surface Preparation
The first and probably most important step of surface preparation is cleaning. Contaminants such as oils, greases, flux, dust, and corrosion by-product can negatively impact the contact between the substrate and the adhesive, thus reducing the capacity of the adhesive to saturate the surface. Before any subsequent surface treatments, all impurities should be removed from the surface as a first step. For instance, by sanding before cleaning any oils or grease on a porous surface, the contaminants may reach deeper into the substrate and cause adhesion problems once the oil outgasses to the surface. When choosing the correct cleaning agents, both the substrate material and source of contamination need to be considered. Organic solvents such as ketones are great against polar contaminants such as flux salts; however, they can potentially eat away certain thermoplastics causing them to soften or haze. MG Chemicals has an extensive line of cleaners that are suitable for a variety of different substrates and contamination sources.
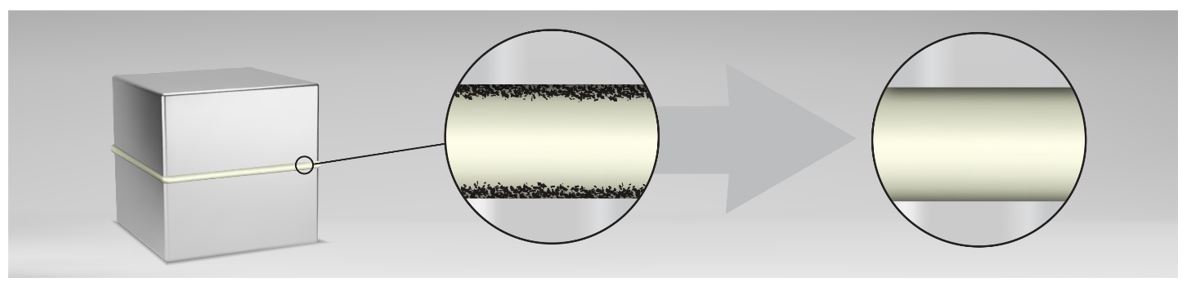
In some cases, cleaning alone is not enough to provide a suitable surface for bonding. In those cases, it is necessary to treat the surface with different methods, which we can classify as; mechanical, chemical, and physical.
Mechanical Method
Mechanical pre-treatment methods increase the surface area of the substrate, giving the adhesive more contact points to bond with the substrate. The easiest way to increase the surface area is by sanding; however, tumbling and sandblasting are other options when specialized equipment is available. Some drawbacks with mechanical methods are that they are line-of-sight, and any residues generated must be removed from the surface, adding extra time and labour to the process.
Chemical Etching
In this technique, the substrate is immersed in a liquid bath which attacks the surface, creating imperfections like with mechanical methods. With metallic surfaces, acids initiate pitting and generate hydroxide groups along the surface which can covalently bond with the adhesive to form adhesive linkages. Plastics can be etched using strong organic solvents like ketones and esters to increase the surface energy.
Physical Method
Techniques in this method include plasma, flame, and corona treatment. Plasma etching changes the chemical reactivity of atoms in the outermost layer of the substrate, enabling covalent bonding between the surface and adhesive. Flame treatment achieves the same objective but uses a combustion reaction instead of ionized gas to change the chemistry of the outermost surface atoms. Lastly, corona treatment uses a high-frequency corona discharge that breaks oxygen molecules into atomic oxygen, which can chemically react with surface treatments, including coatings and adhesives.
Promoters
Adhesion promoters are substances applied onto the substrate before applying a coating or adhesive. These compounds adhere strongly to the substrate and topcoat but lack the cured properties of the topcoat and thus can’t be used as a stand-alone product. Special consideration must be paid to the substrate type as the efficacy of the adhesion promoter varies between the different surfaces. A silane, for example, is very effective for bonding to glass however is not an ideal adhesion promoter for metal which often uses epoxy or alkyd chemistries as promoters. Formulators of finished products like adhesives and coatings often add adhesion promoters into their formulations to offer turnkey products with primerless adhesion for greater ease of use. MG Chemicals has several turnkey adhesive formulations that bond to several substrates like glass, metal, wood and plastic.
More Information
References
Licari, J. J., & Swanson, D. W. (2016). Adhesives Technology for Electronic Applications: Materials, Processing, Reliability (Materials and Processes for Electronic Applications) (2nd ed.). William Andrew.
Handbook of Adhesives and Sealants (McGraw-Hill Handbooks) by Edward Petrie (2007–01-02). (2022). McGraw-Hill Education.
Petrie, E. (2010). Epoxy Adhesive Formulations. McGraw-Hill Professional Publishing.
